|
MERS-CoV potential animal reservoirs and related bat coronaviruses
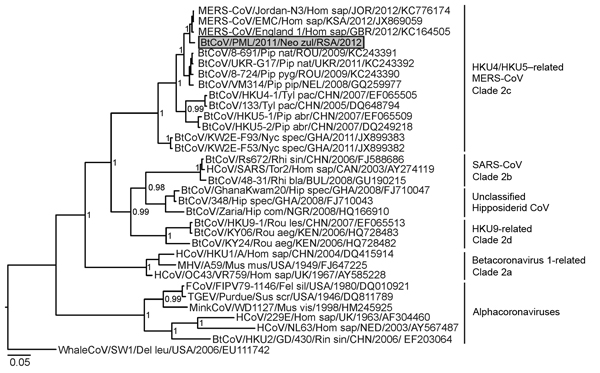
A paper published on Aug 22nd reports a short fragment (182 nucleotides in length) of coronavirus sequence recovered from a sample from an individual Taphozous perforatus or Egyptian tomb bat that was collect a short distance from the home and work location of the first reported case of MERS-CoV infection (Bisha in Western Saudi Arabia). This sequence is reported to be identical across its 182 nucleotides with the same bit of the MERS-CoV genome sequenced from this patient (referred to as EMC-2012).
|
|
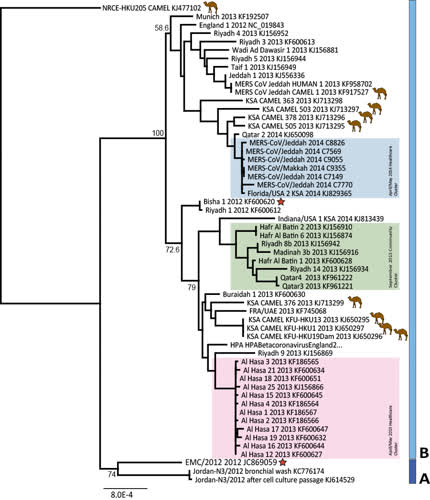
Updated MERS-CoV full genome tree
|
|
MERS-CoV spillover at the camel-human interface
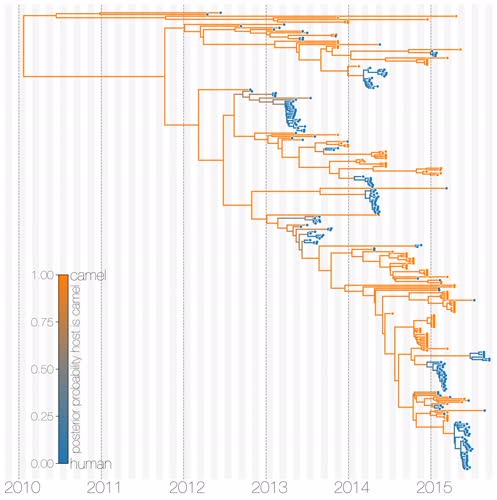
Middle East respiratory syndrome coronavirus (MERS-CoV) is a zoonotic virus from camels causing significant mortality and morbidity in humans in the Arabian Peninsula. The epidemiology of the virus remains poorly understood, and while case-based and seroepidemiological studies have been employed extensively throughout the epidemic, viral sequence data have not been utilised to their full potential. Here, we use existing MERS-CoV sequence data to explore its phylodynamics in two of its known major hosts, humans and camels. We employ structured coalescent models to show that long-term MERS-CoV evolution occurs exclusively in camels, whereas humans act as a transient, and ultimately terminal host. By analysing the distribution of human outbreak cluster sizes and zoonotic introduction times, we show that human outbreaks in the Arabian peninsula are driven by seasonally varying zoonotic transfer of viruses from camels. Without heretofore unseen evolution of host tropism, MERS-CoV is unlikely to become endemic in humans. |