|
The protagonist of this [cytokine] storm is interleukin 6 (IL-6). Features, Evaluation and Treatment Coronavirus (COVID-19) |
|
Covid-10 lowers potassium levels BACKGROUND: SARS-CoV-2 has caused a series of COVID-19 globally. SARS-CoV-2 binds angiotensin I converting enzyme 2 (ACE2) of renin-angiotensin system (RAS) and causes prevalent hypokalemia METHODS: The patients with COVID-19 were classified into severe hypokalemia, hypokalemia, and normokalemia group. The study aimed to determine the relationship between hypokalemia and clinical features, the underlying causes and clinical implications of hypokalemia. RESULTS: By Feb 15, 2020, 175 patients with COVID-19 (92 women and 83 men; median age, 46 [IQR, 34-54] years) were admitted to hospital in Wenzhou, China, consisting 39 severe hypokalemia-, 69 hypokalemia-, and 67 normokalemia patients. Gastrointestinal symptoms were not associated with hypokalemia among 108 hypokalemia patients (P>0.05). Body temperature, CK, CK-MB, LDH, and CRP were significantly associated with the severity of hypokalemia (P<0.01). 93% of severe and critically ill patients had hypokalemia which was most common among elevated CK, CK-MB, LDH, and CRP. Urine K+ loss was the primary cause of hypokalemia. severe hypokalemia patients was given 3 g/day, adding up to an average of 34 (SD=4) g potassium during hospital stay. The exciting finding was that patients responded well to K+ supplements when they were inclined to recovery. CONCLUSIONS: Hypokalemia is prevailing in patients with COVID-19. The correction of hypokalemia is challenging because of continuous renal K+ loss resulting from the degradation of ACE2. The end of urine K+ loss indicates a good prognosis and may be a reliable, in-time, and sensitive biomarker directly reflecting the end of adverse effect on RAS system. |
|
Plenty of coronaviruses but no SARS-CoV-2 separator In our reference institute for infectious diseases, we have been implementing since the end of January 2020 PCR detection of SARS-CoV-2 RNA using several systems, including those released at the European level [4]. In total, we have tested to date (as at 19 February 2020) 4,084 respiratory samples by PCR and all the tests have been negative for SARS-CoV-2. These tests were carried out on the samples of 32 suspected SARS-CoV-2 cases, 337 people repatriated at the beginning of February 2020 from China tested twice, 164 patients who died in public hospitals in Marseille between 2014 and 2019 of whom at least one respiratory sample had been sent to our laboratory, and they also included 3,214 respiratory samples sent since January 2020 to our laboratory to search for a viral aetiology. In striking contrast, we have tested 5,080 respiratory samples for various suspected respiratory viral infections since 1 January 2020 and identified in 3,380 cases respiratory viruses. In decreasing order of frequency, they were: influenza A virus (n = 794), influenza B virus (n = 588), rhinovirus (n = 567), respiratory syncytial virus (n = 361), adenovirus (n = 226), metapneumovirus (n = 192), enterovirus (n = 171), bocavirus (n = 83), parainfluenza virus (n = 24), and parechovirus (n = 8). Among the diagnosed viruses, there were also 373 common human coronaviruses (HCoV), including 205 HCoV-HKU1, 94 HCoV-NL63, 46 HCoV-OC43, and 28 HCoV-229E [5]. Furthermore, analysis of the mortality associated with these viruses has been able to show that since 1 January 2020, one patient died after being diagnosed with HCoV-HKU1, and respiratory viruses were found in 13 other patients who died, which included influenza A virus (3 cases), respiratory syncytial virus (3 cases), rhinovirus (5 cases), adenovirus (1 case) and metapneumovirus (1 case). Retrospectively, analysis of deaths in patients who have had a respiratory sample has shown that at least nine patients have died between 2017 and 2019 after being diagnosed with one of the four coronaviruses commonly circulating in humans [6]. |
|
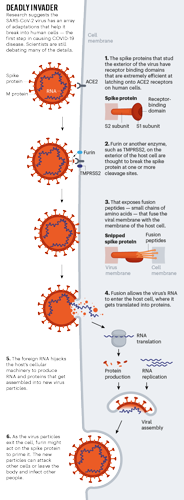
Profile of a killer: the complex biology powering the coronavirus pandemic Coronaviruses are also one of the few RNA viruses with a genomic proofreading mechanism — which keeps the virus from accumulating mutations that could weaken it. That ability might be why common antivirals such as ribavirin, which can thwart viruses such as hepatitis C, have failed to subdue SARS-CoV-2. The drugs weaken viruses by inducing mutations. But in the coronaviruses, the proofreader can weed out those changes.
|
|
ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Damage After 2019-nCoV Infection In December 2019 and January 2020, novel coronavirus (2019-nCoV) - infected pneumonia (NCIP) occurred in Wuhan, and has already posed a serious threat to public health. ACE2 (Angiotensin Converting Enzyme 2) has been shown to be one of the major receptors that mediate the entry of 2019-nCoV into human cells, which also happens in severe acute respiratory syndrome coronavirus (SARS). Several researches have indicated that some patients have abnormal renal function or even kidney damage in addition to injury in respiratory system, and the related mechanism is unknown. This arouses our interest in whether coronavirus infection will affect the urinary and male reproductive systems. Here in this study, we used the online datasets to analyze ACE2 expression in different human organs. The results indicate that ACE2 highly expresses in renal tubular cells, Leydig cells and cells in seminiferous ducts in testis. Therefore, virus might directly bind to such ACE2 positive cells and damage the kidney and testicular tissue of patients. Our results indicate that renal function evaluation and special care should be performed in 2019-nCoV patients during clinical work, because of the kidney damage caused by virus and antiviral drugs with certain renal toxicity. In addition, due to the potential pathogenicity of the virus to testicular tissues, clinicians should pay attention to the risk of testicular lesions in patients during hospitalization and later clinical follow-up, especially the assessment and appropriate intervention in young patients' fertility. |
|
Extrapulmonary manifestations of COVID-19
Although COVID-19 is most well known for causing substantial respiratory pathology, it can also result in several extrapulmonary manifestations. These conditions include thrombotic complications, myocardial dysfunction and arrhythmia, acute coronary syndromes, acute kidney injury, gastrointestinal symptoms, hepatocellular injury, hyperglycemia and ketosis, neurologic illnesses, ocular symptoms, and dermatologic complications. Given that ACE2, the entry receptor for the causative coronavirus SARS-CoV-2, is expressed in multiple extrapulmonary tissues, direct viral tissue damage is a plausible mechanism of injury. In addition, endothelial damage and thromboinflammation, dysregulation of immune responses, and maladaptation of ACE2-related pathways might all contribute to these extrapulmonary manifestations of COVID-19. Here we review the extrapulmonary organ-specific pathophysiology, presentations and management considerations for patients with COVID-19 to aid clinicians and scientists in recognizing and monitoring the spectrum of manifestations, and in developing research priorities and therapeutic strategies for all organ systems involved. |
|
COVID-19: Attacks the1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism The novel coronavirus pneumonia (COVID-19) is an infectious acute respiratory infection caused by the novel coronavirus. The virus is a positive-strand RNA virus with high homology to bat coronavirus. In this study, conserved domain analysis, homology modeling, and molecular docking were used to compare the biological roles of certain proteins of the novel coronavirus. The results showed the ORF8 and surface glycoprotein could bind to the porphyrin, respectively. At the same time, orf1ab, ORF10, and ORF3a proteins could coordinate attack the heme on the 1-beta chain of hemoglobin to dissociate the iron to form the porphyrin. The attack will cause less and less hemoglobin that can carry oxygen and carbon dioxide. The lung cells have extremely intense poisoning and inflammatory due to the inability to exchange carbon dioxide and oxygen frequently, which eventually results in ground-glass-like lung images. The mechanism also interfered with the normal heme anabolic pathway of the human body, is expected to result in human disease. According to the validation analysis of these finds, chloroquine could prevent orf1ab, ORF3a, and ORF10 to attack the heme to form the porphyrin, and inhibit the binding of ORF8 and surface glycoproteins to porphyrins to a certain extent, effectively relieve the symptoms of respiratory distress. Favipiravir could inhibit the envelope protein and ORF7a protein bind to porphyrin, prevent the virus from entering host cells, and catching free porphyrins. Because the novel coronavirus is dependent on porphyrins, it may originate from an ancient virus. Therefore, this research is of high value to contemporary biological experiments, disease prevention, and clinical treatment. |
|
Mutation patterns of human SARS-COV-2 and bat RaTG13 coronaviruses genomes are strongly biased towards C>U indicating rapid evolution in their hosts Background: The world pandemy caused by SARS-CoV-2 spreading has raised considerable interest about its evolutionary origin and genome structure. Here we analysed mutation patterns in 13 human SARS-COV-2 isolates and a closely related RaTG13 isolated from Rhinolophus affinis bat. We also evaluated the CpG dinucleotide contents in SARS-COV-2 and other human and animal coronavirus genomes.
|
|
A ‘silencing’ of pain - SARS-CoV-2 Spike protein co-opts VEGF-A/Neuropilin-1 receptor signaling to induce analgesia lobal spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues unabated. Binding of SARS-CoV-2’s Spike protein to host angiotensin converting enzyme 2 triggers viral entry, but other proteins may participate, including neuropilin-1 receptor (NRP-1). As both Spike protein and vascular endothelial growth factor-A (VEGF-A) – a pro-nociceptive and angiogenic factor, bind NRP-1, we tested if Spike could block VEGF-A/NRP-1 signaling. VEGF-A–triggered sensory neuronal firing was blocked by Spike protein and NRP-1 inhibitor EG00229. Pro-nociceptive behaviors of VEGF-A were similarly blocked via suppression of spontaneous spinal synaptic activity and reduction of electrogenic currents in sensory neurons. Remarkably, preventing VEGF-A/NRP-1 signaling was antiallodynic in a neuropathic pain model. A ‘silencing’ of pain via subversion of VEGF-A/NRP-1 signaling may underlie increased disease transmission in asymptomatic individuals. |
|
Cardiac involvement in 78 patients (78%) and ongoing myocardial inflammation in 60 patients (60%), which was independent of preexisting conditions, severity and overall course of the acute illness, and the time from the original diagnosis.
Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 |
|
Heme; ignore the chloroquine nonsense "That little iron ion, along with millions of its friends released from other hemes, are now floating through your blood freely. As I mentioned before, this type of iron ion is highly reactive and causes oxidative damage. It turns out that this happens to a limited extent naturally in our bodies and we have cleanup & defense mechanisms to keep the balance. The lungs, in particular, have 3 primary defenses to maintain “iron homeostasis”, 2 of which are in the alveoli, those little sacs in your lungs we talked about earlier. The first of the two are little macrophages that roam around and scavenge up any free radicals like this oxidative iron. The second is a lining on the walls (called the epithelial surface) which has a thin layer of fluid packed with high levels of antioxidant molecules.. things like abscorbic [SIC] acid (AKA Vitamin C) among others. Well, this is usually good enough for naturally occurring rogue iron ions but with COVID-19 running rampant your body is now basically like a progressive state letting out all the prisoners out of the prisons… it’s just too much iron and it begins to overwhelm your lungs’ countermeasures, and thus begins the process of pulmonary oxidative stress. This leads to damage and inflammation, which leads to all that nasty stuff and damage you see in CT scans of COVID-19 patient lungs. Ever noticed how it’s always bilateral? (both lungs at the same time) Pneumonia rarely ever does that, but COVID-19 does… EVERY. SINGLE. TIME." |