|
Corporations, Foundations & Organizations
Fiscal Year 2018 Report to Contributors
|
|
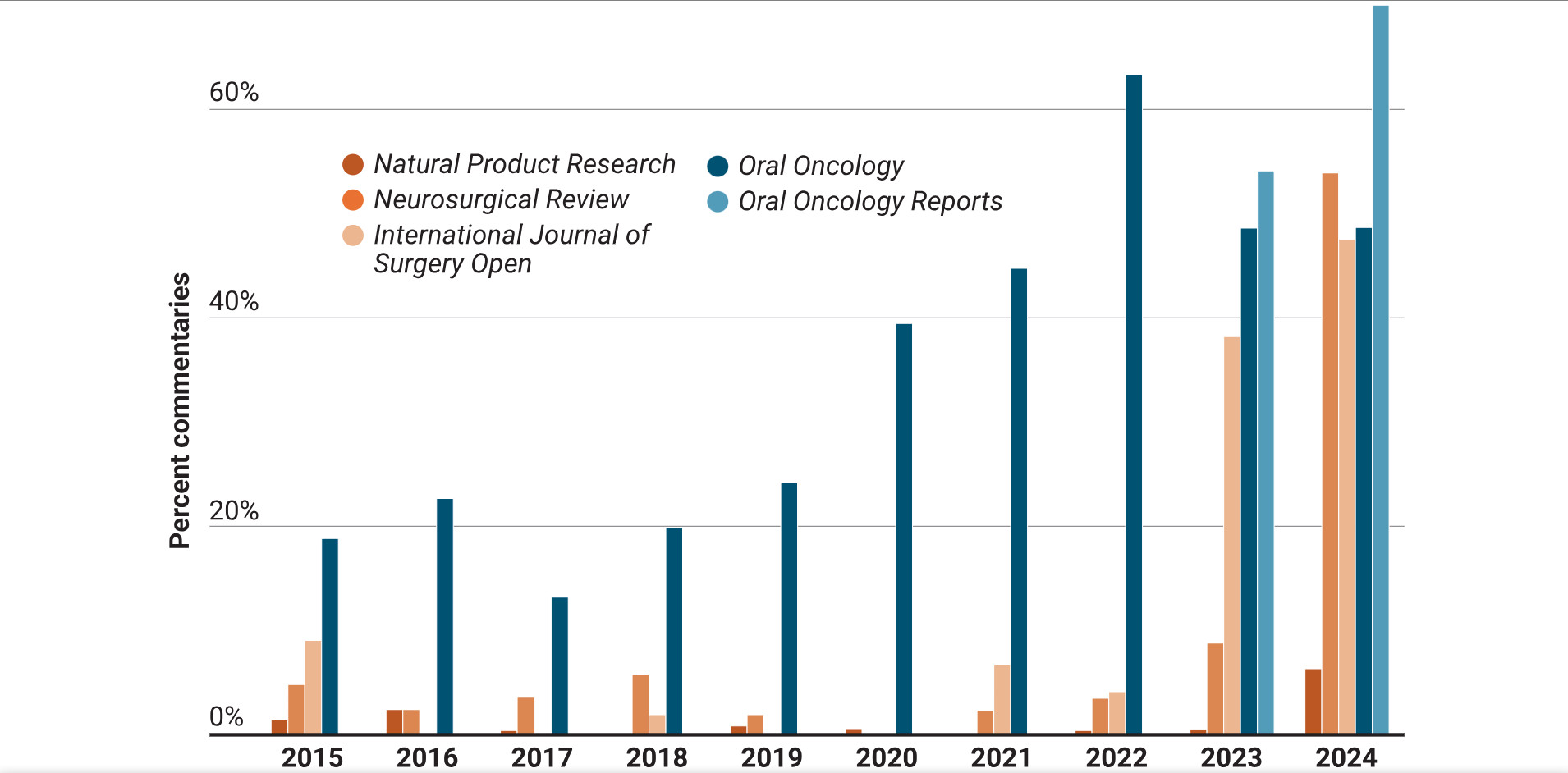
AI-generated content floods literature with poor-quality publications, casts doubt on metrics, Science and Retraction Watch investigation finds
Prestige: verb - Delusion; illusion; trick.
|
|
In 2024 Big Pharma Is Still Making Absurd Profits Off of the Pandemic
Pfizer is projected to rake in more than $50 billion from its COVID medicines this year. Itís a symbol of the drastic inequality created by a for-profit approach to global health.
Last week, drug giant Pfizer told investors that it expects to make more than $50 billion off its COVID-19 medicines this year. Its vaccine is the most lucrative medicine in history, accruing $37 billion in 2021, and has sent its corporate revenues into the stratosphere. By the end of this year, the company hopes to bring in $100 billion ó a sum that exceeds the GDP of most countries on Earth.
Itís been a good pandemic for a company that was, until recently, the least-trusted company in the least-trusted sector in the United States. Not only has the company made a fortune on its COVID medicines, itís also become a household name, with a chief executive who moves among the worldís most powerful leaders, toasted by ordinary people across the world who are desperate for this pandemic to be over. Itís been quite the PR coup.
|
Medical watchdogs reveal how negative data concerning vaccines be marked as false by v non medical "fact checkers" who add enough spin and graphics as to make facts seem obvioulsy false.
Forced to admit in court they're not facts, but are actually "opinions" and no factual claims are made, this did not sit well with thre British Medical Journal (BMJ) who wrote an open letter to Mark Zuckerburg when Facebooks's "fact checkers" incorrectly trashed a scholarly peer reviewed medical journal article.
|
|
|
|
Merck has been so naughty they get their own category.
|
Numbers associated with the problem.
|
Missing clinical trial data must be made public, federal judge says
Feb 2020
Drug companies, device manufacturers, and universities must turn over missing data from hundreds of clinical trials conducted in the United States from 2007Ė17, a federal judge ruled this week. The ruling from the Southern District of New York says government agencies including the National Institutes of Health, the U.S. Department of Health and Human Services, and the Food and Drug Administration (FDA) for years misinterpreted a law requiring them to collect and post data to ClinicalTrials.gov, a publicly accessible government database.
If it is upheld, the ruling would make it harder for drug companies to keep unfavorable results from the public, and it could offer vital information for patients and doctors, STAT reports. Still unclear is how quickly the agencies might move to fill in the 10-year gap in complianceóand what the consequences would be for clinical trial sponsors that donít comply.
A 2015 STAT investigation found that research universities vary widely on how or whether they post clinical trial results. Further reporting by Science found that FDA has never imposed a fine on a clinical trialís sponsor for not complying with federal regulations. The plaintiffs in the lawsuit are Peter Lurie, a former associate FDA commissioner, and Charles Seife, a New York University professor and a writer for multiple outlets, including Science.
|
|
2008 book by Allison Bass
Side Effects: A Prosecutor, a Whistleblower, and a Bestselling Antidepressant on Trial is a nonfiction book by investigative journalist Alison Bass that chronicles the lawsuit filed in 2004 against GlaxoSmithKline by then New York Attorney General Eliot Spitzer.
|
Industry sponsorship and research outcome
Published: 16 February 2017
Authors: Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L
Results from clinical studies on drugs and medical devices affect how doctors practice medicine and thereby the treatments offered to patients. However, clinical research is increasingly sponsored by companies that make these products, either because the companies directly perform the studies, or fully or partially fund them. Previous research has found that pharmaceutical industry sponsored studies tend to favor the sponsorsí drugs more than studies with any other sources of sponsorship. This suggests that industry sponsored studies are biased in favor of the sponsorís products.
This review is an update of a previous review that looked at sponsorship of drug and device studies. The primary aim of the review was to find out whether the published results and overall conclusions of industry sponsored drug and device studies were more likely to favor the sponsorsí products, compared with studies with other sources of sponsorship. The secondary aim was to find out whether such industry sponsored studies used methods that increase the risk of bias, again compared with studies with other sources of sponsorship. In this update, we carried out a comprehensive search of all relevant papers of empirical studies published from 2010 to February 2015 and included 27 new papers, yielding a total of 75 papers included in our review.
|
TAMIFLU FRAUD BILKED $1.5 BILLION FROM GOVERNMENT, ALLEGES WHISTLEBLOWER
JANUARY 13, 2020
Whistleblower Dr. Thomas Jefferson, a physician and public health researcher affiliated with the respected global Cochrane Collaboration research network, has researched neuraminidase inhibitors like Tamiflu for more than two decades. He began questioning Tamifluís efficacy in 2009 and spearheaded efforts to have the company release the underlying clinical study data. When he finally received the data in 2013, Dr. Jefferson analyzed it and concluded that the clinical data does not support Rocheís claims about Tamifluís effectiveness for use in an influenza pandemic, the lawsuit states.
|
|
|